Bromine Monofluoride Uses . Bromine monofluoride (brf) has not been obtained in the pure. With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It reacts with many metals and metal oxides to form similar ionised entities;
from www.chegg.com
It is used in organic chemistry as a fluorinating agent. With some others it forms the metal fluoride plus free bromine and oxygen. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It reacts with many metals and metal oxides to form similar ionised entities; chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. Bromine monofluoride (brf) has not been obtained in the pure.
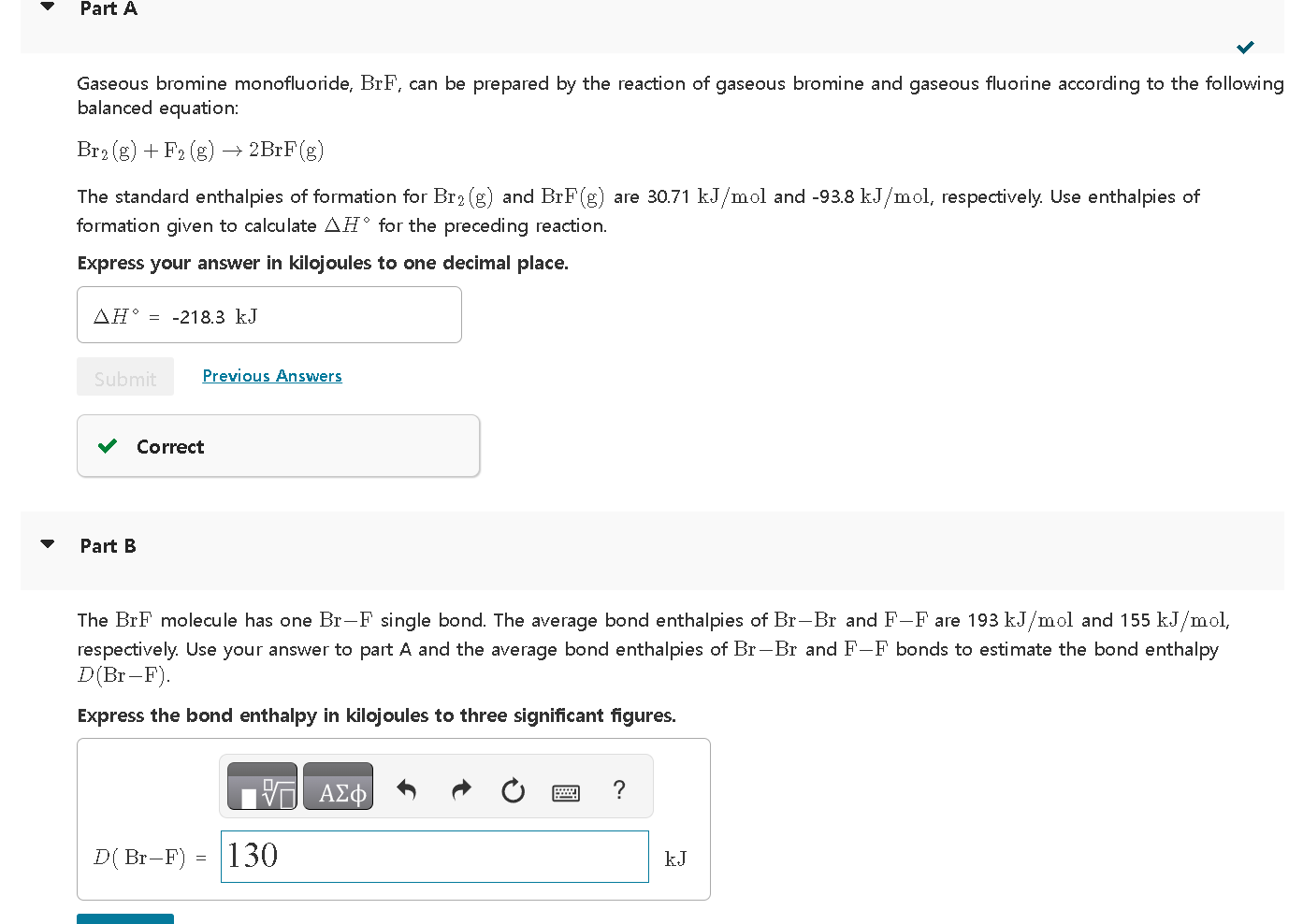
Solved Gaseous bromine monofluoride, BrF, can be prepared by
Bromine Monofluoride Uses chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It reacts with many metals and metal oxides to form similar ionised entities; With some others it forms the metal fluoride plus free bromine and oxygen. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It is used in organic chemistry as a fluorinating agent. Bromine monofluoride (brf) has not been obtained in the pure.
From techiescientist.com
11 Uses of Bromine That You Must Know Techiescientist Bromine Monofluoride Uses It is used in organic chemistry as a fluorinating agent. Bromine monofluoride (brf) has not been obtained in the pure. It reacts with many metals and metal oxides to form similar ionised entities; the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. chlorine monofluoride (clf), the lightest interhalogen, is a colorless. Bromine Monofluoride Uses.
From imgbin.com
Bromine Monofluoride Bromine Pentafluoride Bromine Trifluoride Chlorine Bromine Monofluoride Uses It is used in organic chemistry as a fluorinating agent. It reacts with many metals and metal oxides to form similar ionised entities; the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. With some others it forms the metal fluoride plus free bromine and oxygen. Bromine monofluoride (brf) has not been obtained. Bromine Monofluoride Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Monofluoride Uses the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. With some others it forms the metal fluoride plus free bromine and oxygen. It reacts with many metals and metal oxides to form similar ionised entities; It is used in organic chemistry as a fluorinating agent. chlorine monofluoride (clf), the lightest interhalogen,. Bromine Monofluoride Uses.
From www.chemtube3d.com
BrF Bromine monofluoride Bromine Monofluoride Uses With some others it forms the metal fluoride plus free bromine and oxygen. Bromine monofluoride (brf) has not been obtained in the pure. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It is. Bromine Monofluoride Uses.
From exomkjluk.blob.core.windows.net
Bromine Everyday Uses at Ricky Nesmith blog Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. Bromine monofluoride (brf) has not been obtained in the pure. With some others it forms the metal fluoride plus free bromine and oxygen. chlorine monofluoride (clf), the lightest interhalogen,. Bromine Monofluoride Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation ID2319111 Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; Bromine monofluoride (brf) has not been obtained in the pure. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. With some. Bromine Monofluoride Uses.
From lets-talk-bromine.bsef.com
Bromine. Let’s talk bromine applications, benefits and technologies Bromine Monofluoride Uses It is used in organic chemistry as a fluorinating agent. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It reacts with many metals and metal oxides to form similar ionised entities; the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. Bromine monofluoride. Bromine Monofluoride Uses.
From www.scribd.com
Bromine Monofluoride PDF Chlorine Solution Bromine Monofluoride Uses Bromine monofluoride (brf) has not been obtained in the pure. It reacts with many metals and metal oxides to form similar ionised entities; With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling. Bromine Monofluoride Uses.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Monofluoride Uses chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It is used in organic chemistry as a fluorinating agent. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It reacts with many metals and metal oxides to form similar ionised entities; Bromine monofluoride. Bromine Monofluoride Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID5049252 Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. Bromine monofluoride (brf) has not been obtained in the pure. With some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as. Bromine Monofluoride Uses.
From dic.academic.ru
Фторид брома(I) это... Что такое Фторид брома(I)? Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It is used in organic chemistry as a fluorinating agent. With some others it forms the metal fluoride plus free bromine and oxygen. the bromine monofluoride (brf)’s structural. Bromine Monofluoride Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2813065 Bromine Monofluoride Uses It is used in organic chemistry as a fluorinating agent. Bromine monofluoride (brf) has not been obtained in the pure. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. With some others it forms. Bromine Monofluoride Uses.
From www.chegg.com
Solved The molecule bromine monofluoride, BrF, has a dipole Bromine Monofluoride Uses the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It reacts with many metals and metal oxides to form similar ionised entities; With some others it forms the metal fluoride plus free bromine and oxygen. Bromine monofluoride (brf) has not been obtained in the pure. chlorine monofluoride (clf), the lightest interhalogen,. Bromine Monofluoride Uses.
From www.sliderbase.com
The Halogens Presentation Chemistry Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; It is used in organic chemistry as a fluorinating agent. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. Bromine monofluoride (brf) has not been obtained in the pure. chlorine monofluoride (clf), the lightest interhalogen, is a colorless. Bromine Monofluoride Uses.
From exomkjluk.blob.core.windows.net
Bromine Everyday Uses at Ricky Nesmith blog Bromine Monofluoride Uses Bromine monofluoride (brf) has not been obtained in the pure. It is used in organic chemistry as a fluorinating agent. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. With some others it forms. Bromine Monofluoride Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Monofluoride Uses the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. Bromine monofluoride (brf) has not been obtained in the pure. It reacts with many metals and metal oxides to form similar ionised entities; chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. It is. Bromine Monofluoride Uses.
From ar.inspiredpencil.com
Uses Of Bromine Bromine Monofluoride Uses It reacts with many metals and metal oxides to form similar ionised entities; Bromine monofluoride (brf) has not been obtained in the pure. With some others it forms the metal fluoride plus free bromine and oxygen. the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It is used in organic chemistry as. Bromine Monofluoride Uses.
From wiringdatabaseinfo.blogspot.com
Lewis Dot Diagram For Bromine Wiring Site Resource Bromine Monofluoride Uses the bromine monofluoride (brf)’s structural and electronic properties are widely investigated under high pressure through ab. It reacts with many metals and metal oxides to form similar ionised entities; chlorine monofluoride (clf), the lightest interhalogen, is a colorless gas with a boiling point of 173 °k. Bromine monofluoride (brf) has not been obtained in the pure. With some. Bromine Monofluoride Uses.